Manganese Oxide Phase Diagram

The manganese dioxide is reduced to the manganese oxide hydroxide mno oh during discharging preventing the formation of hydrogen at the anode of the battery.
Manganese oxide phase diagram. Lanthanum strontium manganite lsm or lsmo is an oxide ceramic material with the general formula la 1 x sr x mno 3 where x describes the doping level. A new method for conformal deposition of manganese oxide on high aspect ratio substrates. The perovskite and perovskite derived manganese oxides have attracted considerable interest over the last decade as the richness of their phase diagrams has been uncovered 1 3 phases of interest include a ferromagnetic metallic phase and a paramagnetic phase with short range structural distortions as well as several antiferromagnetic phases. It has a perovskite based crystal structure which has the general form abo 3 in the crystal the a sites are occupied by lanthanum and strontium atoms and the b sites are occupied by the smaller manganese atoms.
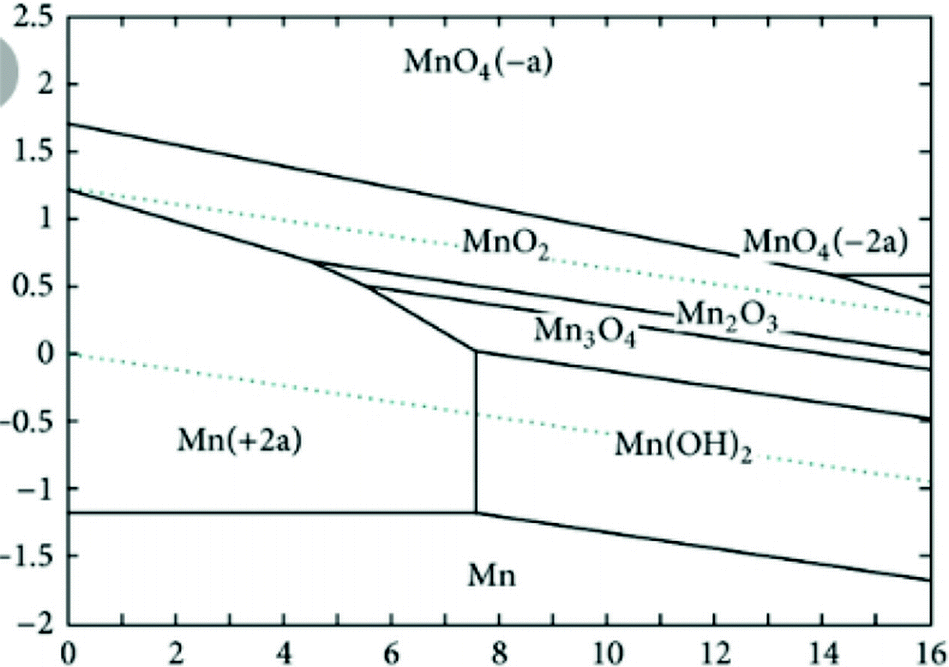
The behavior of manganese in laboratory experiments is in general agreement with predictions of the diagrams. Manganese oxides are one of the most important groups of materials in energy storage science. Here mn 3 o 4 and mn 5 o 8 nanoparticles as well as mesoporous α mn 2 o 3 particles were synthesized by calcination of mn ii. Ib an expanded section of the phase diagram showing the compositions of spinel defect spinel and rock salt structures 25 81.
Bicarbonate and sulfate ions are shown by means of seven stability field diagrams. 3 1 2 the system lix mn2 o4 0 x 2. The calcium manganese oxide materials have a layered structure with considerable thermodynamic stability and a high surface area their low. Manganese iv oxide was used in the original type of dry cell battery as an electron acceptor from zinc and is the blackish material in carbon zinc type flashlight cells.
The dark phase is related to manganese oxide mno while the bright phase represents the tungsten rich phase as confirmed by the eds analysis. A series of calcium manganese oxides ca mn o were prepared through thermal decomposition of carbonate solid solution precursors and investigated as electrocatalysts for oxygen reduction reaction orr. Previous measurements show that calcium manganese oxide nanoparticles are better water oxidation catalysts than binary manganese oxides mn 3 o 4 mn 2 o 3 and mno 2 the probable reasons for such enhancement involve a combination of factors. La the li mn o phase diagram c cubic t tetragonal b limn o4 mno fig.
In order to fully leverage their application potential precise control of their properties such as particle size surface area and mn x oxidation state is required. Li mn2 o4 is a cubic spinel with space group symmetry fd3m. Two loop feynman diagram with quartic vertex. Manganese oxides for lithium batteries mn fig.
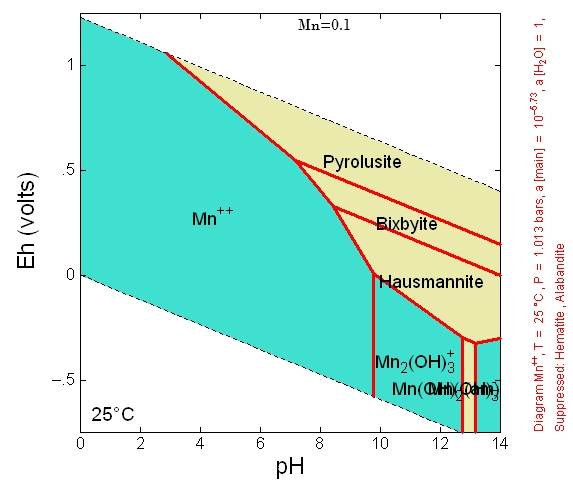
In distilled water with an eh near 0 55 volts and a ph of 7 0 manganese has a solubility of about 1 0 ppm parts per million. Whereas at the three higher temperatures the oxide is found to be as isolated particles.